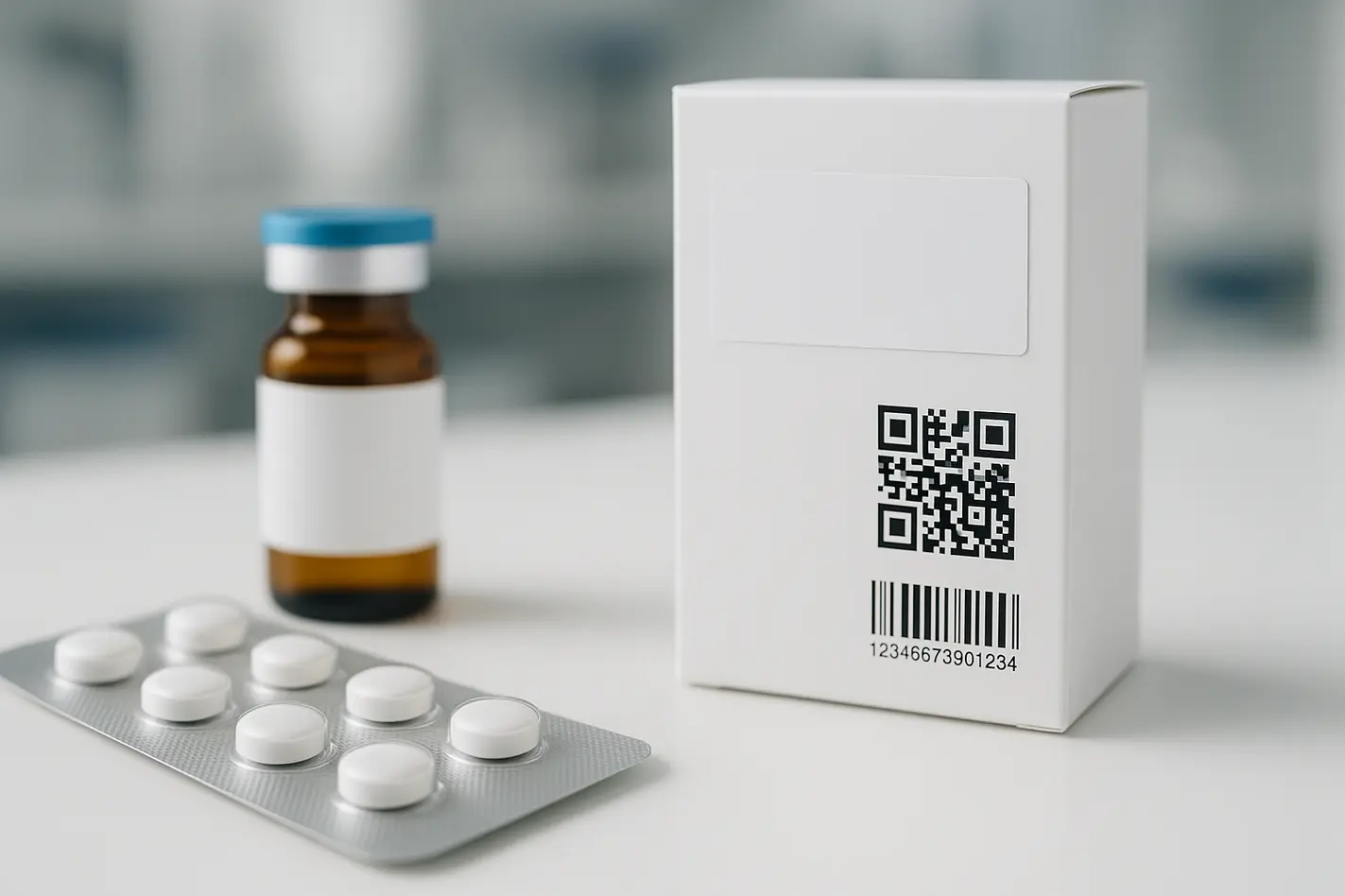
Los envases antifalsificación de medicamentos contribuyen a la seguridad de los pacientes al garantizar que sólo llegan a ellos medicamentos auténticos y seguros. Utiliza dos herramientas clave: códigos de seguimiento únicos (serialización) y señales que indican si alguien ha abierto o modificado el envase (prueba de manipulación). Estas características cumplen las normas legales tanto en la UE como en EE.UU., y contribuyen a la seguridad del paciente, la seguridad del producto y el control de la cadena de suministro.
- Los envases antifalsificación utilizan códigos y sellos exclusivos para proteger los medicamentos de manipulaciones o copias falsas.
- La serialización en el sector farmacéutico hace un seguimiento individualizado de cada envase desde la fábrica hasta la farmacia.
- Los envases a prueba de manipulaciones muestran señales claras si alguien abre o altera el envase.
- Las legislaciones de la UE y EE.UU. exigen características específicas de seguridad y trazabilidad para muchos tipos de medicamentos.
- Un buen diseño también favorece el uso por parte del paciente, como la facilidad de apertura, la claridad de las instrucciones y la resistencia a los niños.
Por qué los medicamentos falsos son un grave problema
Los medicamentos falsificados pueden perjudicar a los pacientes al contener ingredientes erróneos o ninguno en absoluto. Pueden provocar enfermedades, pérdida del efecto del tratamiento e incluso la muerte. También dañan la confianza en el sistema sanitario y crean problemas a las empresas farmacéuticas, que pueden enfrentarse a retiradas del mercado, multas o pérdida de valor de la marca. Las falsificaciones llegan a la cadena de suministro de varias maneras:
•Falsification: Fake medicines that copy real packaging or labels.
•Diversion: Real medicines moved to markets where they are not allowed or priced differently.
•Tampered returns: Used or opened packs sent back and resold without proper checks.
Dado que estos problemas pueden afectar tanto a la seguridad del paciente como a la calidad del producto, los envases antifalsificación son esenciales en la fabricación y distribución de productos farmacéuticos.
Herramientas básicas de la lucha contra la falsificación de envases
Serialización de cada envase
La serialización en farmacia añade un código de barras 2D único a cada envase. Este código de barras incluye la identificación del producto, el número de lote, la fecha de caducidad y un número de serie aleatorio. Las máquinas lo escanean en las fases de producción, envío y farmacia. Esto permite la trazabilidad completa de cada unidad y ayuda a rechazar falsificaciones o verificar devoluciones.
Pruebas de manipulación fáciles de detectar
Los envases a prueba de manipulaciones utilizan precintos, pegatinas o tiras autoadhesivas que muestran si alguien ha abierto el envase. El objetivo no es impedir la apertura, sino mostrarla claramente. El personal sanitario o los pacientes pueden ver si se ha tocado un medicamento y rechazarlo si es necesario. Juntos, la serialización y la prueba de manipulación protegen contra los medicamentos falsos, inseguros o modificados.
Normas de trazabilidad en la UE y EE.UU.
En la UE, la Directiva sobre Medicamentos Falsificados (FMD) establece normas contra la falsificación de envases. La mayoría de los medicamentos con receta deben llevar dos elementos de seguridad: un identificador único y un precinto a prueba de manipulaciones. El identificador se registra en una base de datos nacional y se comprueba antes de dispensar el medicamento para confirmar que el envase es válido.
En Estados Unidos, la Ley de Seguridad de la Cadena de Suministro de Medicamentos (DSCSA) se centra en la serialización y la trazabilidad en todos los socios de la cadena de suministro. Fabricantes, distribuidores y farmacias comparten datos digitales vinculados a cada código. Esto ayuda a identificar productos falsificados o retirados del mercado. También permite comprobar las devoluciones y marcar los envases como inactivos una vez utilizados o retirados.
Aunque los sistemas difieren, ambas leyes pretenden evitar las falsificaciones y proteger la salud pública. Se basan en una codificación coherente, bases de datos precisas y sellos de envasado válidos.
Cómo funciona la tecnología de envasado
Impresión fiable de códigos de barras
La serialización requiere que los códigos estén claramente impresos y sean fáciles de escanear. Utilizamos códigos de barras 2D de alto contraste, legibles por máquina y con un espaciado adecuado. Nuestras cámaras en línea comprueban la claridad y el tamaño de todos los códigos. Una impresión deficiente, los reflejos o una posición incorrecta pueden provocar errores de escaneado o impedir que se acepten los paquetes.
Buen diseño a prueba de manipulaciones
Nuestros formatos de envasado ofrecen precintos y cierres que muestran daños visibles una vez abiertos. Entre ellos se incluyen tiras de rasgado, solapas pegadas o precintos rompibles. El diseño elegido debe ajustarse al tamaño del envase, al tipo de cartón y al uso del paciente. Tras el reenvasado o la inspección, el nuevo precinto también debe demostrar la seguridad con signos visuales claros.
Capas de autenticación inteligentes
Algunos paquetes también incluyen marcadores visibles u ocultos para confirmar la autenticidad. Entre ellos se incluyen:
•Visible features: Color-shifting foils, holograms, or printed patterns.
•Covert markers: Inks or taggants only readable with sensors or apps.
Las herramientas digitales, como las aplicaciones móviles o los dispositivos de escaneado, pueden confirmar la identidad del envase verificando el código con una base de datos central. Esto genera un nivel de confianza adicional.
Agregación de grupos empaquetados
La agregación vincula el código de cada unidad a su grupo mayor, como paquetes o cajas de envío. Una caja maestra puede llevar su propio código que incluya las identidades de todos los paquetes que contiene. Esto simplifica los controles en los almacenes o durante el transporte. Un solo escaneado confirma el contenido de todo el contenedor, lo que ahorra tiempo y mejora la precisión.
Cómo aparecen los riesgos en los envases
Ciertos riesgos pueden provocar fallos en los envases antifalsificación. Estos problemas pueden no ser fáciles de detectar sin herramientas o inspecciones especiales. Los problemas más comunes son:
•Unreadable codes: Caused by poor print quality, incorrect contrast, or glare from packaging material.
•Weak closure: Seals that open too easily or can be closed again without signs of damage.
•Partial updates: Packs that are opened and reworked but not updated in the traceability system.
We use visual inspection systems to scan and confirm each code on the line. Our packaging design and development for risk control includes space planning, seal testing, and layout reviews. All tamper-evident features go through validation to prove they show damage when interfered with.
El comercio paralelo europeo hace que esto sea aún más importante. Cuando un medicamento se reenvasa para un nuevo mercado, el nuevo envase debe tener un código válido y un nuevo cierre a prueba de manipulaciones. Si alguno de los dos falla, el medicamento puede ser rechazado por el sistema.
Algunos medicamentos, como las plumas de inyección o los inhaladores, necesitan un envase adicional. Estos productos combinados conllevan riesgos especiales durante su almacenamiento y necesitan una manipulación cuidadosa. Por ejemplo, un autoinyector debe estar precintado de forma segura, pero ser fácil de abrir en el momento de su uso. Tanto el precinto como las instrucciones deben permanecer claros durante el transporte y el uso.
Cómo apoyamos la lucha contra la falsificación de envases de medicamentos
We provide GMP-level secondary packaging for high-value, sensitive medicines. This includes coding at unit and case level, in-line checks, and data control. Based on product flow and volume, we use in-line printing and sometimes pre-coded cartons. Each method includes inspection to detect defects and confirm compliance.
We also offer child-resistant and tamper-evident designs. These include Locked4Kids packs, which are tested for safety at home. They reduce risk to children while keeping access easy for adults. Our formats show clear opening signs and include guidance for use.
wallets Blister con cold seal frío pueden utilizarse para productos biológicos u otros productos sensibles al calor. El Cold seal frío evita el calor durante el envasado. Esto protege la calidad de los medicamentos y mejora los resultados de impresión. Estas wallets también dejan más espacio para las instrucciones impresas y ayudan a los usuarios a seguir los calendarios correctos.
Hacer más sostenible el envasado seguro
Un buen envase para la trazabilidad de los medicamentos también debe favorecer la sostenibilidad. Utilizamos envases de cartón para reducir el plástico y mejorar la reciclabilidad. Los envases de sellado en frío no utilizan disolventes y son más fáciles de manipular, especialmente para contenidos delicados. Estos envases incorporan elementos de seguridad contra manipulaciones y a prueba de niños sin reducir la seguridad.
A recent case study of ours shows how this works in one product line. It combines usability, safety, and sustainability in one design that fits both patient homes and healthcare settings.
Qué hace que los envases respondan a las necesidades de la industria
Los envases contra la falsificación en el sector farmacéutico deben ofrecer algo más que protección. También debe funcionar en toda la cadena de suministro. Las necesidades clave son:
•High reading success: So barcodes are quickly scanned at all stages.
•Low rejection rates: So safe packs are not wasted due to small errors.
•Clear inspection records: For traceability, audits, and quality control.
•Safe usability: Packs must be easy for adults to open, with clear instructions, and protect children from risk.
Diseñamos y probamos formatos que cumplen estos objetivos, incluidos diseños inteligentes, precintos seguros y datos de inspección completos.
Pasos hacia una mejor elección de envases
Para crear un envase antifalsificación fiable, empiece por determinar el riesgo de cada producto. ¿Es sensible a la temperatura? ¿Existe riesgo de comercio paralelo? ¿Se utiliza en casa por pacientes adultos o en clínicas? En función de estas respuestas, elija características como la serialización, la prueba de manipulación, la resistencia a los niños o la agregación. A continuación, compruebe la calidad de impresión, el manejo por parte del usuario y el escaneado del sistema.
We support this process at every stage. You can also start by using our quick packaging assessment to select the right packaging solution for your product’s needs.
PREGUNTAS FRECUENTES
What is anti-counterfeit packaging for medicines?
Anti-counterfeit packaging helps prevent fake, opened, or misused medicines from reaching patients. It includes unique identification codes and clear proof of opening.
Why is serialization important?
Serialization means every medicine pack gets a unique code, usually in the form of a barcode. This makes it possible to verify that a pack is genuine and allows full traceability in the supply chain. It also supports recalls and returns efficiently.
What does aggregation mean in pharma packaging?
Aggregation connects the unique codes of individual packs with the code of the larger case or carton. When the outer case is scanned, all the inner pack codes are automatically identified. This simplifies logistics, tracking, and quality control.
How is tamper-evident packaging used in practice?
Tamper-evident packaging uses seals, stickers, or flaps that clearly show if a pack has been opened or tampered with. Pharmacists and patients can check these signs before using the medicine to ensure it is safe.
Can anti-counterfeit features affect user experience?
Yes. Security features must be effective but also user-friendly. The design should make codes easy to read and the packaging simple to open, ensuring safety without creating frustration for patients.
Solicite ahora una muestra gratuita